IMARC Group, a leading market research company, has recently released a report titled “Medical Device Outsourcing Market Report by Service (Regulatory Consulting, Product Design and Development, Product Testing and Sterilization, Product Implementation, Product Upgrade, Product Maintenance), Therapeutics (Cardiology, Diagnostic Imaging, Orthopedic, IVD, Ophthalmic, General and Plastic Surgery, Drug Delivery, Dental, Endoscopy, Diabetes Care), Application (Class I, Class II, Class III), and Region 2025-2033”. The study provides a detailed analysis of the industry, including the medical device outsourcing market, growth, size, and industry growth forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
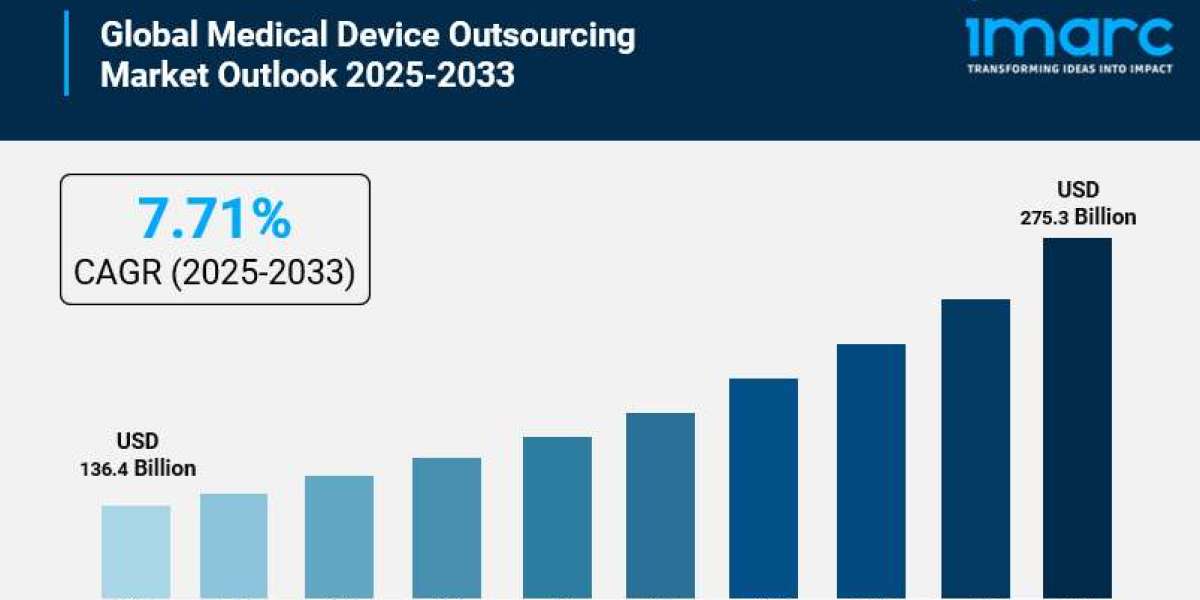
How Big Is the global medical device outsourcing market?
The global medical device outsourcing market size reached USD 136.4 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 275.3 Billion by 2033, exhibiting a growth rate (CAGR) of 7.71% during 2025-2033.
Cost Efficiency and Focus on Core Competencies
The medical device outsourcing market is fueled by the increasing need for cost optimization and the emergence of new opportunities for manufacturers to concentrate on their core competencies such as the innovation of products and clinical research. Through outsourcing various functions like product design, development, regulatory compliance, and manufacturing, medical device companies are able to cut down their overhead expenses and cash in on the shortened time-to-market. The trend of outsourcing is expected to become more pronounced in 2025 as companies will be feeling the heat of high material costs, labor expenses, and regulatory complexities and searching for the most effective ways to solve their problems. Small and mid-sized medical device firms are notably the ones who are relying on outsourcing partners to be able to tap into cutting-edge technology and gain the necessary expertise in the field without having to invest a lot in infrastructure. The move not only makes a significant cost reduction but also increases the scale capability, which then allows businesses to respond to the ups and downs of the market and the changing healthcare demands rapidly. As the competition gets tougher in the industry, outsourcing will be one of the strategic tools that can give a company an edge by enabling it to bring products to the market quickly, make more profits, and focus on patient-driven innovation which is the essence of healthcare.
Increasing Regulatory and Quality Compliance Needs
Global stringent regulatory frameworks are the main factors behind the medical device outsourcing sector development. Meeting standards that are set globally, such as FDA guidelines, ISO certifications, and EU MDR regulations, means that companies have to go through a very thorough documentation, testing, and validation procedure which takes a lot of time and is very expensive. In 2025, the need for those who provide outsourcing regulatory support and quality assurance services will be higher as the standards keep changing and companies have to keep pace. Outsourcing partners that are good in the field of regulations, testing, and quality management systems are becoming medical device manufacturers' go-to solution for ensuring efficient regulatory compliance and at the same time cutting the risk of penalties or product recall. The point is that they must be very cautious in the new territory they are traveling to as the rules are quite different there. The outsourcing approach is going to become more important as a means not only of lessening the alleviation of work loads but also to be able to guarantee quality, safety for the patient, and easy accessibility of markets at a global level.
Access to Advanced Technology and Specialized Expertise
The rapid technological development in 3D printing, robotics, AI-driven diagnostics, and minimally invasive devices is having a considerable impact on the outsourcing landscape. To achieve this, manufacturers will have to depend more and more on the outsourcing providers who are proficient in the state-of-the-art production processes followed by the latest design capabilities. The problem is that many of them, especially the smaller ones, don't have the money to do that kind of work inside. This is the reason why outsourcing is the best answer for that problem. This trend is also fuelled by the interesting and complicated nature of medical devices that call for the employment of experts from different fields: material science, software integration, etc. Outsourcing service providers that are continuously on the path of innovation are a company's go-to resource for achieving the competitive edge in launching differentiated and high-performance products. In addition, outsourcing allows easier business growth, equipping societies with the tools to manage sudden increases in demand without quality being compromised. Through employing partners who are technologically capable, medical device firms can stay at the front of the pack and roll out products that meet both clinical and consumer expectations.
Medical Device Outsourcing Market Trends 2025
The medical device outsourcing market is changing radically with the combining of the three factors: cost efficiency, technology adoption and regulatory support. The market trend in 2025 that would have the biggest influence on it would be the growing move up from stand-alone deals to the establishment of long-term strategic partnerships. The practice of manufacturers working together with outsourcing providers not only for production but also for diverse end-to-end services that cover product design, clinical trial management, and post-market surveillance is rapidly increasing. Communication facilitated by digital technologies, such as cloud-based platforms for data sharing and AI-driven analytics, is enhancing the effectiveness of companies and their partners and also reducing the scope of errors. Also, sustainability is becoming an important factor as outsourcing partners are investing in eco-friendly materials, green manufacturing processes, and waste reduction initiatives to align with global environmental goals. The growing need for customized and connected medical devices, such as wearable health monitors and smart implants, is also compelling outsourcing partners to make their devices more adaptable and innovative. Altogether, these trends present the picture that outsourcing is one of the major means through which medical device companies are able to survive and thrive in a competitive and highly regulated industry.
Get your Sample of Medical Device Outsourcing Market Insights for Free: https://www.imarcgroup.com/medical-device-outsourcing-market/requestsample
Industry Segmentation:
Analysis by Service:
- Regulatory Consulting
- Product Design and Development
- Product Testing and Sterilization
- Product Implementation
- Product Upgrade
- Product Maintenance
Analysis by Therapeutics:
- Cardiology
- Diagnostic Imaging
- Orthopedic
- IVD
- Ophthalmic
- General and Plastic Surgery
- Drug Delivery
- Dental
- Endoscopy
- Diabetes Care
Analysis by Application:
- Class I
- Class II
- Class III
Regional Insights:
- North America
- Europe
- Asia Pacific
- Middle East and Africa
- Latin America
Who are the key players operating in the industry?
The report covers the major market players including:
- Celestica Inc. (Onex Corporation)
- Charles River Laboratories International Inc.
- Flex Ltd.
- Freyr Inc.
- Heraeus Holding GmbH
- ICON plc
- Integer Holdings Corporation
- IQVIA Inc.
- Plexus Corp.
- Sanmina Corporation
- TE Connectivity
- West Pharmaceutical Services Inc.
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/request?type=report&id=5468&flag=E
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302