IMARC Group, a leading market research company, has recently released a report titled "Drug-Eluting Stents Market Report by Coating (Polymer Based Coating, Polymer Free Coating), Drug (Sirolimus, Paclitaxel, Zotarolimus, Everolimus, Biolimus, and Others), Stent Platform (Stainless-steel, Cobalt-Chromium, Platinum-Chromium, Nitinol, and Others), Generation (1st Generation, 2nd Generation, 3rd Generation, 4th Generation), Application (Coronary Artery Disease, Peripheral Artery Disease), End User (Hospitals, Ambulatory Surgical Centers, and Others), and Region 2025-2033." The study provides a detailed analysis of the industry, including the global drug-eluting stents market size, trends, share and growth forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Drug-Eluting Stents Market Highlights:
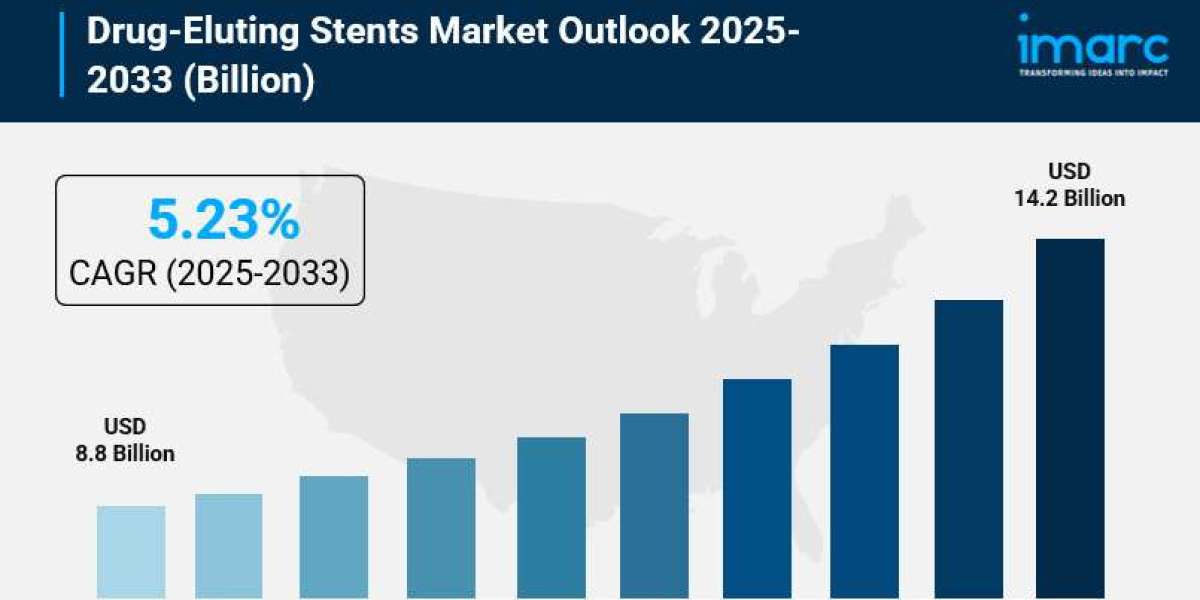
- Drug-Eluting Stents Market Size: Valued at USD 8.8 Billion in 2024.
- Drug-Eluting Stents Market Forecast: The market is expected to reach USD 14.2 billion by 2033, growing at an impressive rate of 5.23% annually.
- Market Growth: The drug-eluting stents market is experiencing robust growth driven by rising cardiovascular disease prevalence and advancing medical technologies.
- Technology Integration: Next-generation drug formulations like biolimus and zotarolimus are revolutionizing treatment outcomes with enhanced efficacy and reduced adverse effects.
- Regional Leadership: North America commands the largest market share, supported by advanced healthcare infrastructure and widespread adoption of cutting-edge stent technology.
- Application Expansion: Beyond coronary applications, drug-eluting stents are increasingly being utilized for peripheral artery disease treatment, expanding their therapeutic scope.
- Key Players: Industry leaders include Abbott, Medtronic plc, Boston Scientific Corporation, and Cook Medical, which dominate the market with innovative solutions.
- Market Challenges: Complex regulatory approvals and the need for continuous technological advancement to address diverse patient populations present ongoing challenges.
Claim Your Free “Drug-Eluting Stents Market” Insights Sample PDF: https://www.imarcgroup.com/drug-eluting-stents-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends and Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Industry Trends and Drivers:
- Revolutionary Next-Generation Drug Integration:
The cardiovascular device industry is witnessing a transformative shift with the implementation of advanced drug formulations in stent technology. Modern drug-eluting stents are incorporating sophisticated medications like biolimus and zotarolimus, which demonstrate significantly higher efficacy in preventing restenosis while minimizing adverse effects. This technological leap is making these devices suitable for a broader spectrum of patients with varying cardiovascular conditions. In May 2024, Abbott announced the launch of XIENCE Sierra Everolimus (drug) eluting coronary stent system in India. It is a CE-marked and one of the latest generation stents in the XIENCE family, now available to people suffering from blocked coronary arteries. This advancement represents a significant milestone in interventional cardiology, setting new benchmarks for treatment outcomes.
- Expanding Applications Beyond Coronary Care:
Drug-eluting stents are experiencing unprecedented expansion into peripheral artery disease treatments, moving well beyond their traditional coronary applications. This diversification reflects the medical community's growing confidence in stent technology for treating blockages in arteries throughout the body, not just around the heart. In April 2024, Cook Medical received a contract to supply implantable devices to hospitals run by the U.S. Department of Defense (DoD). The company would provide its Zilver PTX drug-eluting peripheral stent, Zenith aortic endograft, and other implantable devices. This strategic expansion is opening new revenue streams while addressing previously underserved patient populations suffering from peripheral vascular conditions.
- Enhanced Capabilities for Complex Cardiovascular Cases:
Healthcare providers are increasingly turning to drug-eluting stents for treating the most challenging cardiovascular cases, including complex coronary lesions such as bifurcation and multi-vessel disease. These advanced stents are specifically engineered to handle intricate anatomical challenges with unprecedented precision and reliability. In June 2024, Elixir Medical's DynamX Sirolimus-Eluting Coronary Bioadaptor System received Breakthrough Device Designation from the FDA. This bioadaptive implant aims to improve coronary luminal diameter, restore hemodynamic modulation, and reduce plaque progression in patients with symptomatic ischemic heart disease. This FDA recognition underscores the industry's commitment to addressing the most demanding clinical scenarios.
- Rising Global Cardiovascular Disease Burden:
The worldwide increase in cardiovascular diseases is creating substantial demand for advanced interventional solutions. Aging populations combined with lifestyle-related risk factors such as obesity, smoking, and sedentary behavior are contributing to a surge in cardiovascular interventions. According to the World Health Organization, cardiovascular diseases remain the leading cause of death globally, affecting millions of people annually. This epidemiological trend is driving healthcare systems to invest heavily in proven technologies like drug-eluting stents that can effectively reduce the need for repeat procedures while improving patient outcomes. The growing awareness of cardiovascular health and improved diagnostic capabilities are also contributing to earlier detection and treatment, further expanding the market demand.
Drug-Eluting Stents Market Report Segmentation:
Breakup by Coating:
- Polymer-Based Coating
- Polymer Free Coating
Breakup by Drug:
- Sirolimus
- Paclitaxel
- Zotarolimus
- Everolimus
- Biolimus
- Others
Breakup by Stent Platform:
- Stainless-steel
- Cobalt-Chromium
- Platinum-Chromium
- Nitinol
- Others
Breakup by Generation:
- 1st Generation
- 2nd Generation
- 3rd Generation
- 4th Generation
Breakup by Application:
- Coronary Artery Disease
- Peripheral Artery Disease
Breakup by End User:
- Hospitals
- Ambulatory Surgical Centers
- Others
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
Who are the key players operating in the industry?
The report covers the major market players including:
- Abbott
- B. Braun SE
- Biosensors International Group, Ltd.
- Biotronik
- Boston Scientific Corporation
- Cook Medical
- Lepu Medical Technology (Beijing) Co., Ltd
- Medinol
- Medtronic plc
- MicroPort Scientific Corporation
- Sino Medical Sciences Technology Inc
- Terumo Corporation
Ask Analyst For Request Customization: https://www.imarcgroup.com/request?type=report&id=4815&flag=E
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1–201971–6302