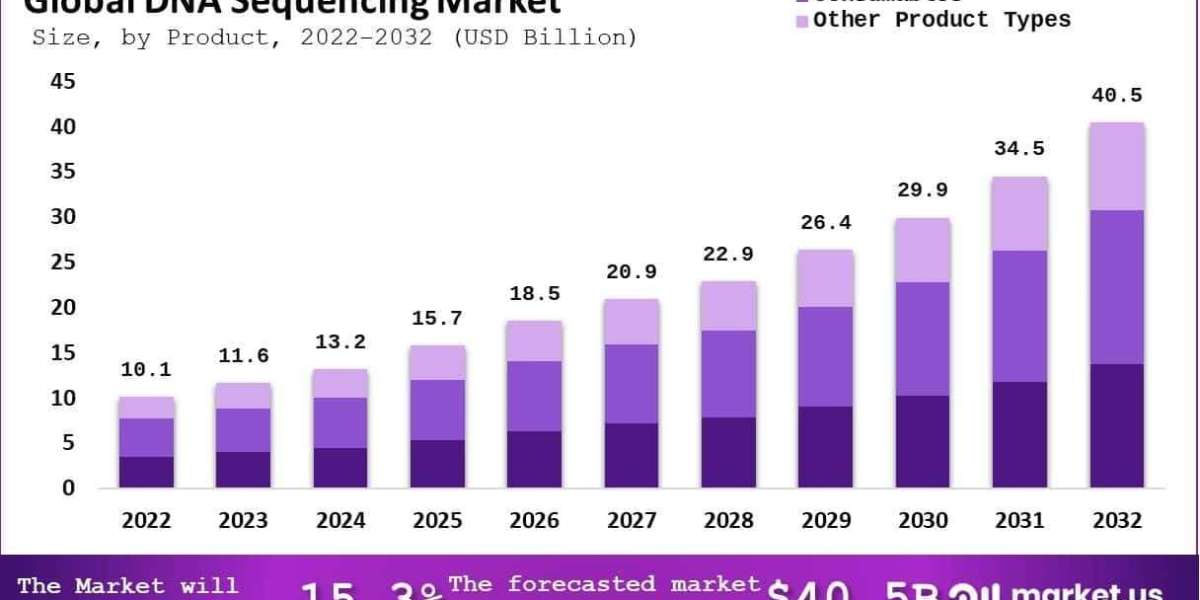
The Global DNA Sequencing Market size is expected to be worth around USD 40.5 Billion by 2033, from USD 10.1 Billion in 2023, growing at a CAGR of 15.3% during the forecast period from 2024 to 2033.
In 2025, the DNA Sequencing Market is gaining momentum as clinical-grade platforms integrate into wide-reaching precision medicine initiatives. Hospitals and diagnostic labs are implementing rapid sequencing protocols for oncology, rare genetic disorders, and transplant compatibility. Point-of-care sequencing devices now deliver actionable results in under 24 hours, helping clinicians rapidly tailor treatments.
Pharma companies are leveraging real-world sequencing data to monitor drug resistance and support targeted therapy approvals. As healthcare systems roll out reimbursement for clinically validated genomic tests, DNA sequencing is moving from research-centric to a standard-of-care tool—enabling truly personalized diagnostics and treatment planning.
Click here for more information: https://market.us/report/dna-sequencing-market/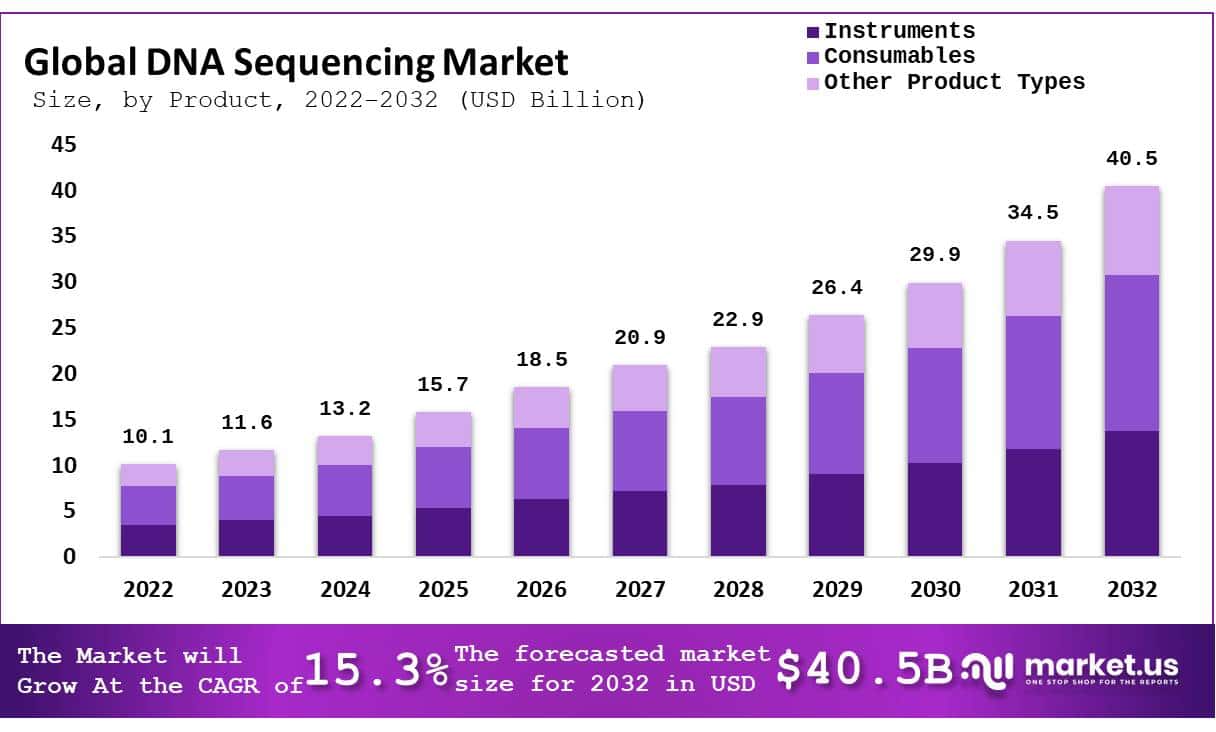
Key Market Segments
Based on Product
- Instruments
- Consumables
- Other Product Types
Based on Sequencing Type
- Next-generation Sequencing
- Sanger Sequencing
- Other Sequencing Types
Based on Application
- HLA Typing
- Oncology
- Clinical Investigation
Based on End-User
- Academics & Research Institutions
- Pharmaceutical & Biotechnology Companies
- Hospitals & Healthcare Organizations
- Other End Users
Emerging Trends
- Point-of-care clinical sequencers delivering results in less than 24 hours.
- Integration of sequencing data into EHRs for routine tumor boards and genetic counseling.
- Real-world drug-resistance monitoring via population sequencing dashboards.
- Insurance reimbursement frameworks supporting clinically validated genomic tests.
Use Cases
- Oncologists use rapid tumor exome sequencing to refine chemotherapy within 48 hours.
- A transplant team sequences donor-recipient HLA mismatches at bedside for faster decisions.
- A pharma company monitors tuberculosis genetic resistance across global patient cohorts.
- A rare-disease clinic integrates newborn genome sequencing results into care planning.