The Global Amyotrophic Lateral Sclerosis Treatment Market was valued at USD 600 Million in 2022 and expected to grow USD 1021 Million in 2032. Between 2023 and 2032, this market is estimated to register a CAGR of 5.6%.
In 2025, the ALS Treatment Market is on the cusp of transformation with the rise of neuroprotective small molecules and gene-modifying therapies. Landmark drugs targeting SOD1 and C9orf72 mutations are entering late-stage trials and showing slowed neuronal degeneration. Innovative delivery platforms—like intrathecal viral vectors and lipid nanoparticles—are enabling targeted treatment with fewer systemic side effects.
With regulatory bodies offering accelerated approval pathways for high unmet needs, pharmaceutical firms are forging partnerships to bring mutation-specific therapies to global patients. As personalized medicine drives treatment decisions, ALS care is shifting from palliative focus to disease-altering strategies.
Click here for more information: https://market.us/report/amyotrophic-lateral-sclerosis-als-treatment-market/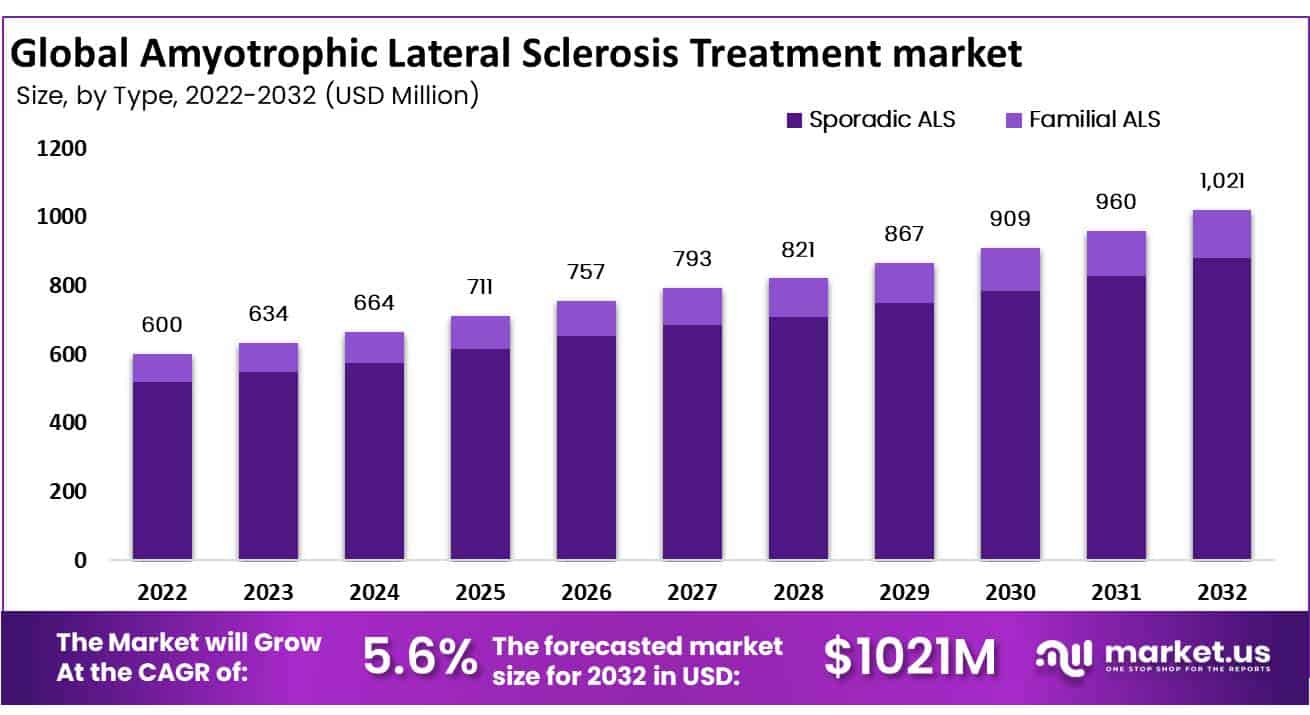
Emerging Trends
- Antisense oligonucleotides (ASOs) administered intrathecally to silence specific genetic mutations.
- Small-molecule neuroprotectants designed to stabilize glutamate excitotoxicity and oxidative stress.
- Lipid nanoparticle vectors for safer systemic delivery of gene therapies.
- Biomarker-guided trial enrollment, using neurofilament light levels and imaging to track progression.
Use Cases
- A C9orf72 patient enrolls in an ASO trial, showing slowed decline in motor function after a year.
- Early-stage patients receive a neuroprotective agent that delays respiratory support milestones by six months.
- A biotech company vets LNP-based gene delivery to reduce side effects in ALS mouse models.
- Biomarkers like NfL are used to stratify trial participants for better outcome correlation.



