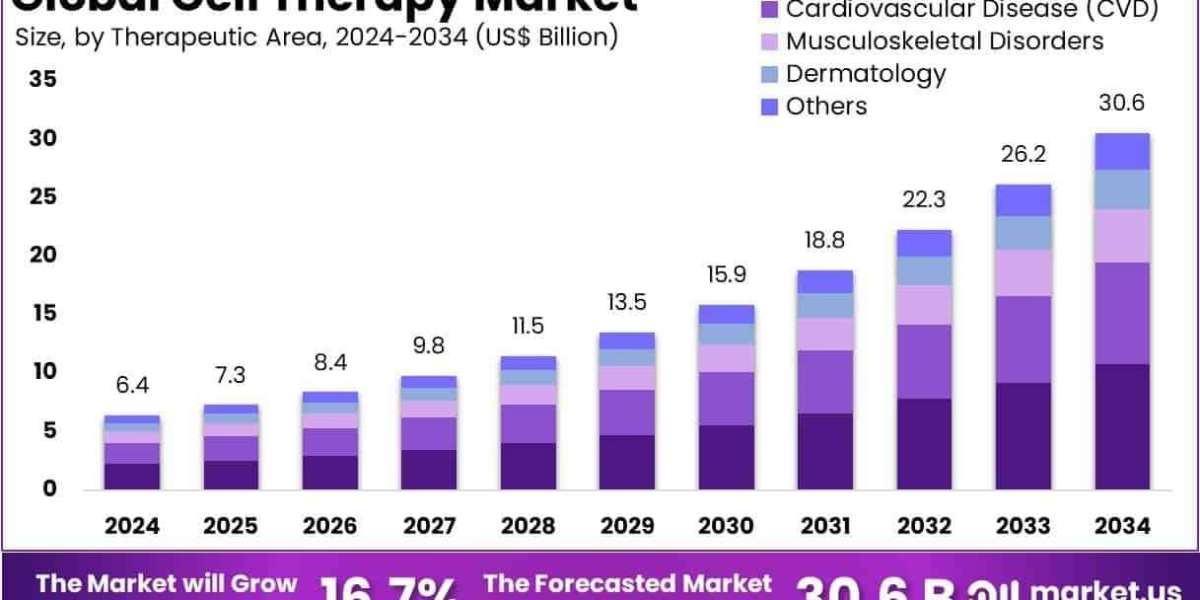
The Global Cell Therapy Market size is expected to be worth around US$ 30.6 Billion by 2034, from US$ 6.4 Billion in 2024, growing at a CAGR of 16.7% during the forecast period from 2025 to 2034. North America held a dominant market position, capturing more than a 53.1% share and holds US$ 3.3 Billion market value for the year.
In 2025, the Cell Therapy Market is accelerating thanks to breakthroughs in allogeneic “off-the-shelf” products and more efficient manufacturing. These therapies—derived from donor cells—offer immediate availability and cost efficiencies over traditional autologous approaches. Generics and biotech firms are scaling up using single-use bioreactors and automated cell culture systems, enabling large batch production.
Regulatory agencies are adapting guidelines to support these universal products. As hospitals and ambulatory centers prepare for on-demand cell therapies, patient access and affordability are both improving. This approach is expected to expand the market beyond cancer to include autoimmune and regenerative medicine, positioning cell therapy as a mainstream treatment modality.
Click here for more information: https://market.us/report/cell-therapy-market/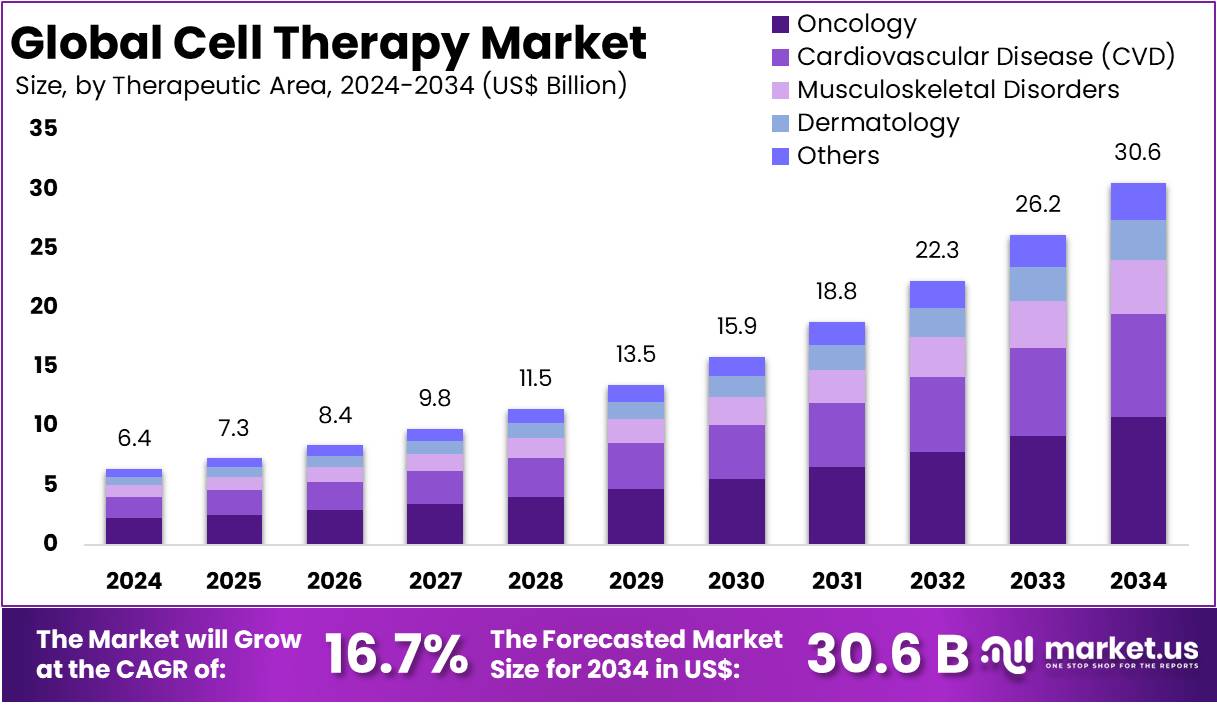
Emerging Trends
- Off‑the‑shelf allogeneic CAR T/NK products reducing wait time and cost.
- Automated cell expansion platforms streamlining batch quality and consistency.
- Regulatory frameworks evolving to standardize universal donor products.
- Satellite manufacturing hubs bringing production closer to point of care.
Use Cases
- A hospital receives frozen allogeneic CAR‑NK cells and treats acute myeloid leukemia within 48 hours.
- A CDMO implements automated cell culture to produce hundreds of CAR T doses in one run.
- Regulators fast‑track approval for a universal CAR T product with standard safety profile.
- A regional clinic uses a local cell-processing lab to avoid long transport times for patient treatments.