IMARC Group has recently released a new research study titled “United States Gene Therapy Market Size, Share, Trends and Forecast by Gene Type, Vector Type, Delivery Method, Application, and Region, 2025-2033”, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
Market Overview
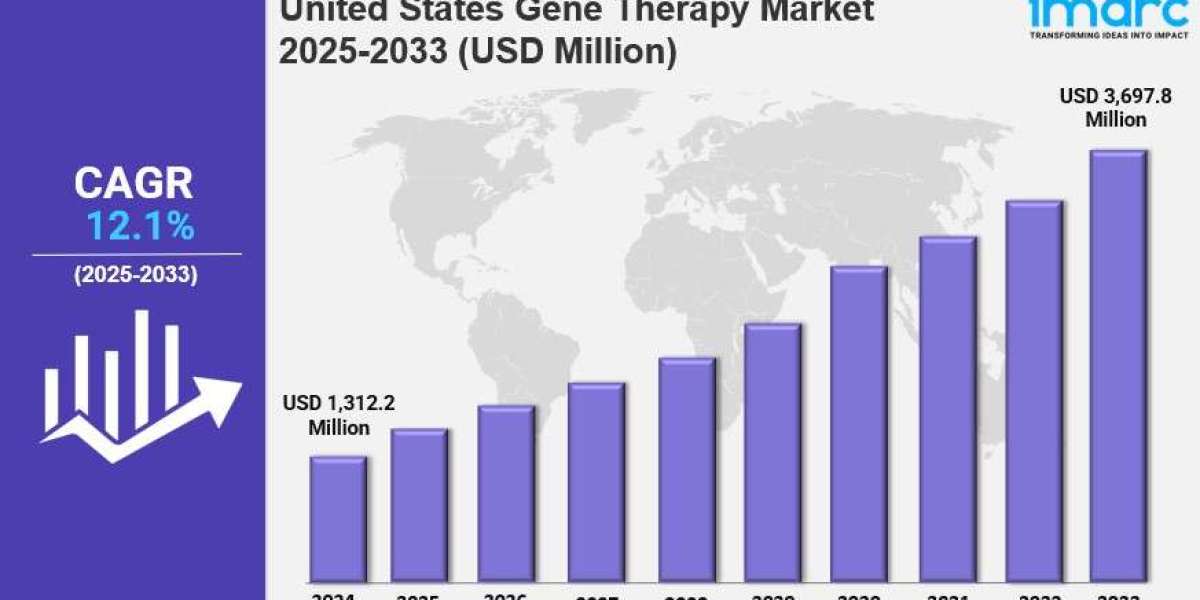
The United States gene therapy market size was valued at USD 1,312.2 Million in 2024. It is forecasted to reach USD 3,697.8 Million by 2033, exhibiting a CAGR of 12.1% for the period 2025–2033. Key growth drivers include increasing prevalence of genetic disorders, rapid genetic engineering advancements, strategic collaborations, supportive regulatory frameworks, and rising R&D investments. The market evolution is also supported by expanding healthcare infrastructure and improved insurance coverage enabling greater patient access.
Study Assumption Years
- Base Year: 2024
- Historical Year/Period: 2019-2024
- Forecast Year/Period: 2025-2033
United States Gene Therapy Market Key Takeaways
- Current Market Size: USD 1,312.2 Million (2024)
- CAGR: 12.1%
- Forecast Period: 2025-2033
- The United States has designated over 1,000 orphan drugs including many gene therapies under FDA orphan drug programs.
- FDA approvals for gene therapies have more than doubled since 2017, with over 20 clearances by 2023.
- More than 100 gene therapies received the FDA's RMAT designation by 2023, accelerating approvals.
- Healthcare infrastructure supports over 50 certified centers and 114 gene therapy trials started in 2023.
- Insurance coverage is increasingly favorable, exemplified by Novartis's coverage of Zolgensma for 97% commercial and 86% Medicaid patients.
Sample Request Link: https://www.imarcgroup.com/united-states-gene-therapy-market/requestsample
Market Growth Factors
The United States gene therapy market demand is driven by the increasing prevalence of genetic disorders and chronic diseases, particularly cancer, along with growing investments in advanced therapeutic research and development. In 2024, the U.S. is expected to diagnose around 2,001,140 new cancer cases, with approximately 611,720 deaths. The aging population, projected to increase by 47% from 58 million in 2022 to 82 million by 2050, drives demand for more effective therapies like gene therapy, especially for cancer treatment that precisely targets malignant cells to reduce damage.
Technological improvements in genetic engineering and biotechnology, such as CRISPR/Cas9 and other gene-editing tools, have enhanced the detection and correction of genetic mutations. In October 2023, NIH awarded nearly USD 40 million to Yale School of Medicine for developing a phase 2 CRISPR-based gene therapy targeting genetic brain diseases, reflecting increased R&D investments accelerating clinical trials.
Strategic collaborations and partnerships are boosting the gene therapy industry’s growth in the U.S. Examples include the January 2023 exclusive agreement between Spark Therapeutics and Neurochase to develop Neurochase's CNS delivery technology with Spark's AAV platform. Such collaborations pool resources and expertise to overcome gene therapy development challenges, facilitating innovation and expanding treatment options.
To get more information on this market Request Sample
Market Segmentation
Analysis by Gene Type:
- Antigen: Enhances immune system ability to recognize and fight diseases like cancer by producing antigens aiding immunotherapy.
- Cytokine: Involves genes that regulate inflammation and immune responses, crucial for cancer and chronic inflammatory conditions.
- Tumor Suppressor: Introduces or restores genes to inhibit uncontrolled cell growth, addressing genetic mutations suppressing tumors.
- Suicide Gene: Designed to selectively cause death of targeted cells, primarily cancer cells, minimizing damage to healthy tissue.
- Deficiency: Targets correction of genetic disorders by inserting functional genes, addressing inherited diseases like cystic fibrosis.
- Growth Factors: Supports cell growth and tissue regeneration, managing degenerative disorders and trauma-related recovery.
- Receptors: Modifies or adds genes coding for receptors to enhance cancer cell targeting via immune cell modification.
- Others
Analysis by Vector Type:
- Viral Vector: Includes adenoviruses, lentiviruses, retroviruses, adeno-associated virus, herpes simplex virus, poxvirus, vaccinia virus, and others; widely used for efficient gene delivery with stable expression.
- Non-Viral Techniques: Naked and plasmid vectors, gene gun, electroporation, lipofection, and others; provide lower immunogenicity and more flexible genetic modification.
Analysis by Delivery Method:
- In-Vivo Gene Therapy: Genetic material is directly delivered into the patient's body to induce therapeutic effects through vectors like viral particles.
- Ex-Vivo Gene Therapy: Patient’s cells are modified outside the body and then reintroduced, allowing controlled modification and testing prior to implantation.
Analysis by Application:
- Oncological Disorders: Gene therapies target and eliminate tumors by manipulating genes in immune or cancer cells.
- Rare Diseases: Addresses inherited genetic disorders lacking conventional treatments, e.g., hemophilia and spinal muscular atrophy.
- Cardiovascular Diseases: Tests genes improving heart muscle or new blood vessel development and regulating genetic factors in CVDs.
- Neurological Disorders: Focuses on inherited neurodegenerative diseases, delivering targeted gene solutions inside the central nervous system.
- Infectious Disease: Employs genes that enhance immune responses or create antiviral proteins to combat infections.
- Others
Regional Insights
The gene therapy market in the United States is well distributed with key regional hubs. The Northeast is a major center due to prestigious universities, biotech clusters, and research facilities, driving innovation through partnerships. The West region hosts a high concentration of pharmaceutical and biotech companies supported by venture capital and collaborations. The Midwest and South are growing, supported by investments, biotech firms, and life sciences programs. Specific market share or CAGR by region is not provided in the source. Overall, these regions collectively foster the country's gene therapy ecosystem.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=11240&flag=C
Recent Developments & News
In November 2024, PTC Therapeutics, Inc. announced FDA accelerated approval for its gene therapy treating aromatic L-amino acid decarboxylase (AADC) deficiency, the first US-approved gene therapy administered directly to the brain. In April 2024, Pfizer Inc. received FDA approval for BEQVEZ (fidanacogene elaparvovec-dzkt), an adeno-associated virus vector-based gene therapy for adults with moderate to severe hemophilia B, specifically for patients without neutralizing antibodies to AAVRh74var capsid. These developments highlight ongoing regulatory progress.
Competitive Landscape
The competitive landscape of the industry has also been examined along with the profiles of the key players.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No: (D) +91 120 433 0800
United States: +1-201971-6302