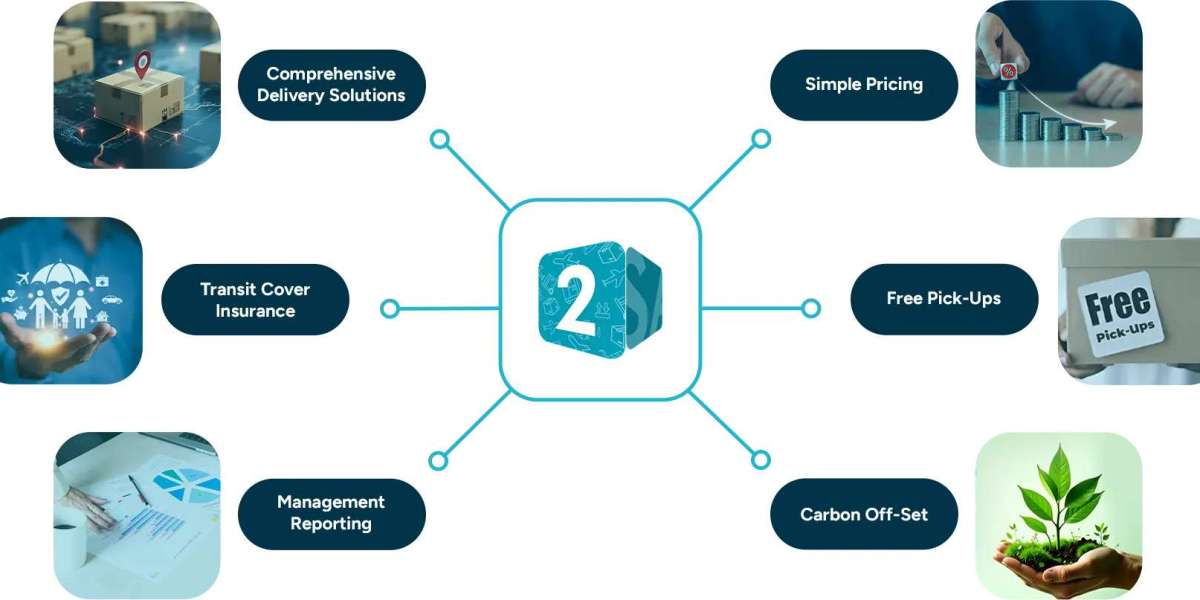
In today's fast-moving world of ecommerce and international trade, choosing the right courier service Australia-wide can make all the difference for both businesses and everyday senders. With customers expecting faster delivery times, transparent tracking, and cost-efficient services, reliability is now a non-negotiable feature. Ship2Anywhere has become a trusted name in this space, offering dependable solutions for parcel delivery Australia-wide and international shipments. Its commitment to technology, service variety, and transparent communication is what sets it apart in a competitive logistics market.
One of the reasons businesses continue to choose a reputable courier service Australia-wide is the consistency it brings to each stage of the shipping process. Ship2Anywhere focuses heavily on efficiency, from providing instant quotes to integrating premium delivery partners across the globe. This ensures customers receive top-tier service whether they're sending a local parcel or arranging express shipping to Australia from overseas suppliers.
Speed is a crucial factor, particularly for ecommerce sellers and businesses managing time-sensitive shipments. Ship2Anywhere offers reliable express shipping to Australia that connects international senders with Australian customers faster and more affordably. Its multi-carrier system compares leading courier networks, allowing customers to select the most suitable express option based on price, delivery window, and destination. This flexibility makes express shipments more accessible to growing brands looking to scale globally without facing overwhelming logistics costs.
Another major advantage is transparency. Today's consumers want full visibility over their deliveries, and Ship2Anywhere's platform supports detailed, real-time tracking for all parcel delivery Australia orders. Whether a parcel is moving through domestic hubs or arriving from an international location, customers are kept informed at every stage. This reduces uncertainty, improves customer satisfaction, and helps businesses avoid unnecessary service enquiries.
Reliability also stems from the range of services offered. Not all parcels are alike, and different customers have different needs. Ship2Anywhere supports domestic parcel services, international freight, business shipping solutions, and express shipping to Australia for urgent deliveries. This variety empowers both individuals and businesses to choose the right service without compromising on timelines or pricing.
Behind every trusted courier service Australia wide lays strong customer support. Ship2Anywhere prides itself on providing approachable, responsive assistance for questions related to booking, tracking, customs requirements, or delivery expectations. This hands-on support helps customers navigate challenges smoothly, especially when dealing with cross-border shipping.
With smart technology, dependable partnerships, and service flexibility, Ship 2 Anywhere continues to strengthen its position as a reliable delivery option for parcel delivery in Australia and international express services. Its focus on affordability, transparency, and customer satisfaction ensures that senders have full confidence every time they ship locally or globally.